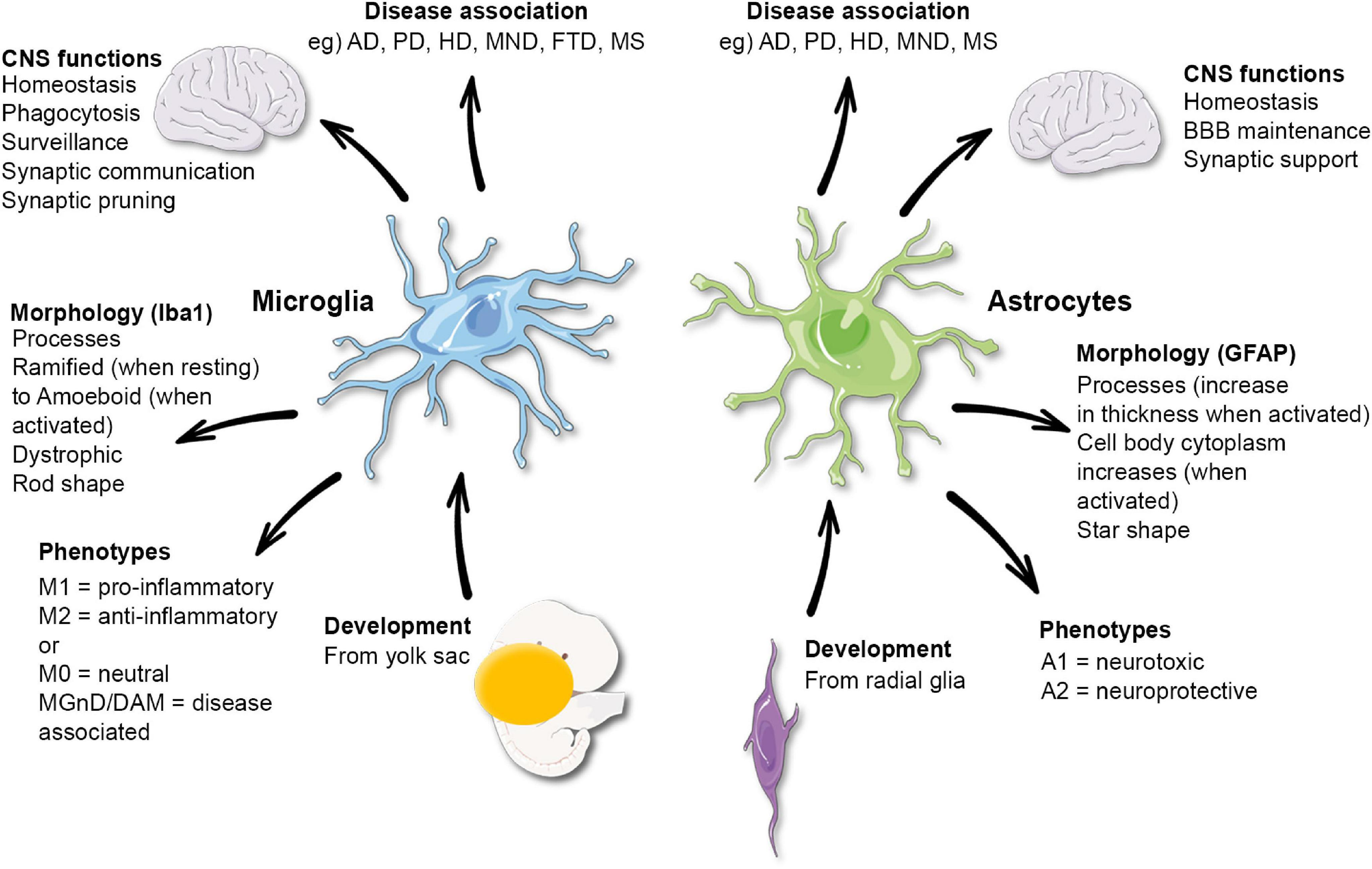
Microglial research is at the forefront of neuroscience, revealing critical insights into the brain’s immune system and its role in neurodegenerative diseases like Alzheimer’s disease. These specialized cells, known as microglia, continuously monitor brain health by removing damaged neurons and engaging in synaptic pruning, a process vital for proper brain function. Recent studies led by prominent researchers, including Beth Stevens, have highlighted how dysfunctional microglial activity can contribute to the progression of various disorders, significantly impacting our understanding of Alzheimer’s. By exploring the mechanisms behind microglial function, scientists are uncovering potential new biomarkers and therapies that may alter the course of neurodegenerative diseases. This innovative research promises to enhance the lives of millions grappling with these debilitating conditions.
Research into microglial cells—often termed the brain’s immune defenders—has revolutionized our comprehension of neurological health and disease. These unique glial cells play a fundamental role in maintaining brain homeostasis, offering insights into the pathology of ailments such as Alzheimer’s disease and other neurodegenerative conditions. Investigators like Beth Stevens have unveiled the intricate relationship between these immune cells and processes such as synaptic pruning, which while essential for development, can sometimes lead to adverse outcomes if misregulated. Through this lens, the quest to understand how microglial dysfunction can lead to disease progression has opened new avenues for innovative therapies and diagnostic tools needed to address these growing health concerns. This burgeoning field underscores the interconnectedness of immune responses within the brain and their implications for human health.
Understanding Microglial Cells and Their Role in Alzheimer’s Disease
Microglial cells are critical components of the brain’s immune system, playing an essential role in maintaining neural health. These specialized cells continuously monitor the environment and respond to potential threats, including pathogens and cellular debris. Recent studies by neuroscientists, including Beth Stevens, emphasize that while microglial activation can have protective effects, it can also lead to detrimental outcomes like excessive synaptic pruning, a process that has been linked to neurodegenerative diseases such as Alzheimer’s. Enhanced microglial activity might result in the loss of vital synapses, which are necessary for communication between neurons, ultimately impairing cognitive functions.
Understanding the dual nature of microglial cells is key to deciphering their contribution to Alzheimer’s disease progression. As researchers like Beth Stevens delve deeper into this field, they reveal the critical balance that microglia must maintain in response to neural damage or disease. Ongoing microglial research is aimed at uncovering the pathways that govern their behavior, with the hope that targeted interventions can either enhance their protective functions or inhibit harmful activities, thus providing new avenues for Alzheimer’s treatment.
The Impact of Synaptic Pruning on Neurodegenerative Diseases
Synaptic pruning is a crucial process in brain development and function, allowing for the elimination of excess synapses to refine neural circuits. However, the misregulation of this process can lead to severe implications, particularly in neurodegenerative diseases like Alzheimer’s and Huntington’s. Studies conducted by the Stevens Lab highlight that abnormal synaptic pruning by microglial cells may contribute to cognitive decline and memory loss associated with these conditions. This underscores the importance of understanding how microglia interact with neuronal networks and their role in the pathology of diseases that affect millions.
The connection between synaptic pruning and neurodegenerative diseases is becoming increasingly emphasized in research. By exploring the mechanisms through which microglia determine which synapses to prune, researchers are identifying potential biomarkers and therapeutic targets. As we learn more about the implications of synaptic pruning on brain health, particularly in the context of Alzheimer’s disease, it becomes evident that interventions aimed at regulating microglial activity could play a transformative role in developing effective treatments.
The Future of Neurodegenerative Disease Research
The landscape of neurodegenerative disease research is rapidly evolving, driven by discoveries related to the brain’s immune system. Pioneers in the field, such as Beth Stevens, are pushing the boundaries of our understanding of diseases like Alzheimer’s by investigating the intricate relationships between microglia, synapses, and neuronal health. With recent advances in technology and methodology, researchers are now able to visualize and manipulate microglial activity in unprecedented ways, providing new insights into their role in maintaining brain homeostasis.
Looking ahead, the future of neurodegenerative disease research appears promising, with a focus on translating basic science discoveries into clinical applications. The path laid by foundational research will guide the development of targeted therapies that aim to correct aberrant microglial behavior, enhance neuroprotection, and potentially reverse cognitive decline in conditions like Alzheimer’s disease. As the scientific community consolidates its efforts, the goal remains clear: to improve the lives of millions affected by these debilitating conditions through innovative research.
Beth Stevens: A Pioneer in Neuroimmunology
Beth Stevens stands out as a pioneer in the burgeoning field of neuroimmunology, particularly with her groundbreaking work on microglial cells. Her research has significantly advanced our understanding of how these immune cells interact with neurons and how their dysfunction can lead to neurodegenerative diseases such as Alzheimer’s. As a recipient of the MacArthur Fellowship, Stevens has been recognized not just for her scientific achievements but also for her vision of how microglial research can lead to practical therapeutic advances.
Stevens’s approach combines fundamental science with potential clinical applications, seamlessly bridging the gap between laboratory research and real-world impact. Her insights into the role of microglia in synaptic pruning have opened new avenues for understanding neurodegenerative diseases. By fostering an environment where curiosity-driven research can flourish, Stevens exemplifies the importance of foundational studies in ultimately paving the way for innovations in treatment and prevention of illnesses like Alzheimer’s.
Synaptic Pruning and Cognitive Health in Alzheimer’s
Cognitive health in Alzheimer’s patients is intricately linked to the process of synaptic pruning, primarily mediated by microglial cells. Under normal circumstances, synaptic pruning is critical for the optimal functioning of neural networks, but its dysregulation can result in significant cognitive impairment. Recent studies indicate that excessive pruning of synapses by hyperactive microglia may disrupt the neural connections necessary for memory and learning, thus accelerating the progression of Alzheimer’s disease.
Investigating the precise mechanisms underlying synaptic pruning provides valuable insights into Alzheimer’s pathology. By elucidating how microglia mistakenly prune healthy synapses, researchers can develop strategies to modulate this process, aiming to restore balance and protect cognitive functions. Continued research in this area not only enhances our understanding of synaptic dynamics but also points towards novel therapeutic targets that could mitigate the effects of Alzheimer’s and other neurodegenerative diseases.
The Role of Microglia in Synaptic Development
Microglia play a critical role in synaptic development during early brain formation, guiding the maturation and remodeling of neural circuits. They are essential for shaping the architecture of the brain by removing unnecessary synapses, thereby optimizing the efficiency of neural signaling. Understanding the dynamics of microglial activity can shed light on how disruptions during this developmental phase might lead to cognitive disorders later in life, including Alzheimer’s disease.
Research led by Beth Stevens focuses on characterizing the signals that prompt microglia to prune synapses effectively in healthy brains. By investigating these processes, Stevens aims to identify how alterations in microglial function might contribute to neurodegenerative diseases. This foundational research is crucial, as it lays the groundwork for discovering potential therapeutic strategies that could help restore normal synaptic pruning processes in Alzheimer’s disease and improve overall brain health.
Towards Innovative Therapies for Neurodegenerative Disorders
The quest for innovative therapies for neurodegenerative disorders is gaining momentum, especially as researchers like Beth Stevens continue to unveil the complexities of microglial function in diseases such as Alzheimer’s. Understanding how microglia contribute to both neuroprotection and neurodegeneration offers promising avenues for intervention. The insights gleaned from Stevens’s work on synaptic pruning provide a vital framework for developing medications that can either enhance the beneficial effects of microglia or inhibit their harmful actions.
As novel therapeutic approaches are devised, the integration of discoveries in microglial research will be paramount. By translating these findings into clinical practice, scientists aim to provide effective treatments that not only address symptoms but also modify underlying disease progression. Moving forward, the emphasis will be on collaborative efforts within the scientific community to harness basic research breakthroughs into practical solutions that improve outcomes for those living with neurodegenerative diseases.
The Intersection of Immunology and Neurology
The intersection of immunology and neurology has become a focal point in understanding neurodegenerative diseases, particularly as researchers explore the role of the brain’s immune cells—microglia. The application of immunological principles to neurologic conditions has revealed the complex interactions between immune responses and neural health. With Beth Stevens as a leading figure in this research domain, the implications for diseases like Alzheimer’s are profound, as microglia are now recognized as key players in both inflammatory responses and neurodevelopment.
Exploring this intersection not only aids in understanding the mechanisms of neurodegeneration but also has the potential to reshape treatment paradigms. By targeting microglial dysfunction, researchers hope to develop strategies that restore immune balance in the brain, ultimately leading to improved outcomes for patients with Alzheimer’s disease. The synergy of immunology and neurology represents a promising frontier in medical research, emphasizing the need for continued exploration of these connections.
The Importance of Funding in Neuroscience Research
Funding plays a pivotal role in advancing neuroscience research, particularly in the area of neurodegenerative diseases. As highlighted by Beth Stevens, much of her groundbreaking work on microglia and their implications in diseases like Alzheimer’s stems from support by federal agencies such as the National Institutes of Health. This funding not only facilitates the exploration of fundamental scientific questions but also allows researchers to advance towards practical applications that may one day alleviate the burden of these conditions.
The importance of sustained investment in scientific research cannot be overstated. With increased funding, researchers can undertake more comprehensive studies, explore innovative hypotheses, and ultimately contribute to a more profound understanding of diseases. As microglial research continues to evolve, adequate financial support will remain essential in translating discoveries into meaningful treatments, emphasizing the need for advocacy and policy formulation that prioritizes funding in neuroscience.
Frequently Asked Questions
How do microglial cells influence Alzheimer’s disease through synaptic pruning?
Microglial cells act as the brain’s immune system, constantly monitoring for signs of injury or disease. In Alzheimer’s disease, abnormal synaptic pruning by these cells can lead to excessive removal of synapses, contributing to cognitive decline. Understanding this process is crucial in developing therapeutic strategies aimed at restoring normal microglial function and preventing synaptic loss.
What role does Beth Stevens play in advancing microglial research related to neurodegenerative diseases?
Beth Stevens, a prominent neuroscientist, is at the forefront of microglial research, particularly in relation to Alzheimer’s disease and other neurodegenerative conditions. Her studies have revealed how microglia’s role in synaptic pruning can lead to dysfunction in neurological health, providing insights into potential biomarkers and treatments for these diseases.
Why are microglial cells important in the brain’s immune system, especially in the context of neurodegenerative diseases?
Microglial cells are essential components of the brain’s immune system, as they protect brain health by removing dead cells and debris. In neurodegenerative diseases like Alzheimer’s, the malfunction of microglia can exacerbate disease progression, making them a focus for research aimed at understanding and treating these conditions.
What are some potential implications of microglial research for Alzheimer’s disease treatments?
Research on microglial cells has significant implications for Alzheimer’s disease treatments by identifying new biomarkers and therapeutic targets. By understanding how microglial dysfunction contributes to disease progression, researchers like Beth Stevens aim to develop strategies to normalize microglial activity and improve cognitive function in affected individuals.
How has foundational research in microglial studies changed our understanding of neurodegenerative diseases?
Foundational research in microglial studies has transformed our understanding of neurodegenerative diseases by revealing the crucial role of microglial cells in synaptic pruning and brain health. This body of work has laid the groundwork for breakthrough discoveries in Alzheimer’s disease and other conditions, paving the way for innovative treatments.
What does the future hold for microglial research in the context of neurodegenerative disorders?
The future of microglial research in neurodegenerative disorders looks promising, with ongoing studies focused on the mechanisms behind microglial dysfunction. Researchers are optimistic that unraveling these mechanisms may lead to the development of effective therapies for diseases like Alzheimer’s, ultimately improving patient outcomes.
| Key Point | Details |
|---|---|
| Importance of Microglia | Microglia serve as the brain’s immune system, monitoring for illness or injury. |
| Role in Alzheimer’s Disease | Abnormal microglial pruning can lead to neurodegenerative diseases like Alzheimer’s and Huntington’s. |
| Research Impact | Stevens’ lab is pioneering new biomarkers and potential treatments for neurodegenerative diseases. |
| Funding Sources | Research heavily supported by the NIH and federal grants, demonstrating the value of foundational science. |
| Future Directions | Continued exploration of immune pathways in the brain could reveal crucial insights into treating human diseases. |
Summary
Microglial research is essential in unlocking the secrets of neurodegenerative conditions such as Alzheimer’s disease. By exploring how microglial cells impact synaptic pruning and brain health, researchers like Beth Stevens are paving the way for innovative treatments and better diagnostics for millions affected by these debilitating disorders. Understanding the complex role of microglia informs future studies and potential therapies, emphasizing the importance of foundational research in advancing our grasp of brain health.