Patient Safety in Medical Research is paramount in ensuring that individuals participating in clinical trials receive the utmost protection during their involvement in studies. The integrity of medical research relies heavily on robust oversight mechanisms, such as Institutional Review Boards (IRBs) that guarantee compliance with ethical standards and regulations. As federal funding, including NIH funding, faces substantial obstacles, concerns grow over how these cuts may impact the rights of research participants and their overall safety. The ability to conduct thorough evaluations of research proposals and mitigate potential risks is jeopardized, which could lead to ethical violations and harm to patients. Ultimately, the commitment to maintaining high standards in medical research is foundational to public trust and the advancement of innovative healthcare solutions.
Ensuring the well-being of individuals involved in health-related studies is critical in the context of clinical experimentation. Safety measures are not merely administrative necessities but are foundational to the ethical conduct of research involving human subjects. Institutional oversight, often provided by dedicated committees such as IRBs, plays a vital role in evaluating research initiatives and safeguarding participant rights. As the landscape of medical investigation is influenced by factors such as funding fluctuations, especially from major sources like the NIH, the implications for patient safety in pharmaceutical trials and medical devices become increasingly significant. The historical lessons reiterate the need for stringent measures to be in place, fostering an environment where clinical research can thrive without compromising the dignity and safety of its participants.
The Impact of Funding Cuts on Patient Safety in Medical Research
The recent suspension of over $2 billion in federal research grants can severely undermine the integrity and safety of patient participation in medical research. Funding from organizations like the NIH plays an essential role in maintaining robust oversight through Institutional Review Boards (IRBs), which ensure that medical studies comply with ethical standards and government regulations. These boards are vital in protecting participants’ rights, monitoring their safety, and ensuring informed consent processes are meticulously followed. When funding is halted, IRBs may lack the resources necessary to conduct thorough reviews of research proposals, leading to potential oversights in participant safety protocols.
Moreover, as research funding decreases, the ability to engage with multiple research sites concurrently is compromised. This creates bottlenecks in clinical trials that not only elongate timelines but may also overlook critical patient safety measures. For example, without adequate funding to facilitate the SMART IRB system’s operations, collaborative research efforts face delays that can result in participants remaining unobserved for safety and well-being. By jeopardizing patient oversight, the funding freeze, therefore, risks eroding public trust in the research process—a trust that is paramount for encouraging enrollment in clinical trials.
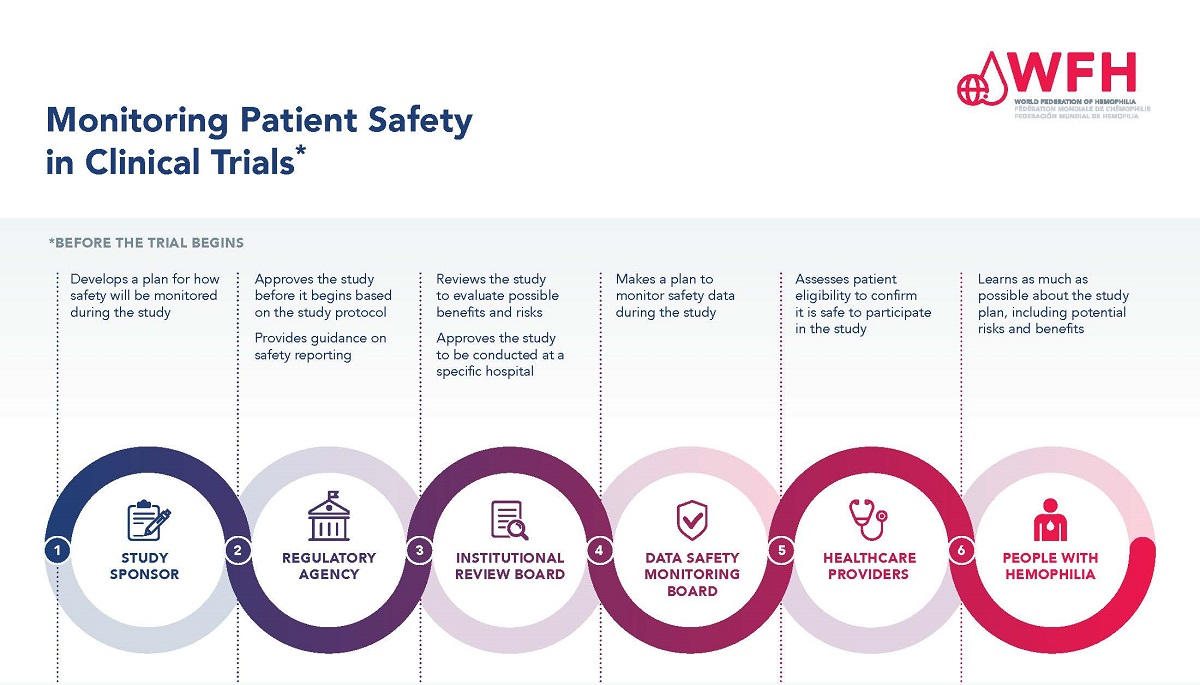
Understanding the Role of IRBs in Medical Research
Institutional Review Boards (IRBs) are critical components of the medical research landscape, functioning as the ethical guardians of research participants. Their main responsibility is to evaluate research proposals to ensure that they adhere to ethical guidelines and legal standards, prioritizing participants’ rights and safety. IRBs dictate rigorous oversight processes, ensuring that research studies are structured to minimize risks to participants and that informed consent is comprehensively communicated and understood. The IRBs’ role underscores an essential dimension in safeguarding research participant rights, particularly in collaborative and multisite studies.
The oversight provided by IRBs is not just bureaucratic; it is a foundational element celebrating the progress of ethical medical research. Historical malpractice demonstrates the catastrophic consequences when such oversight is lacking. Events now seen through the lens of ethical scrutiny, such as the Tuskegee Syphilis Study, highlight the necessity for an accountable system that institutions like IRBs embody. They ensure ongoing engagement with research participants, thereby fostering an environment of transparency and trust. Without robust IRB oversight, there could be a resurgence of unethical practices which would directly endanger the rights and well-being of those who volunteer for clinical research.
The Crucial Nature of Transparent Communication in Clinical Trials
Transparent communication within clinical trials serves as a cornerstone for building trust between researchers and participants. For individuals considering participation in a study, having access to accurate and comprehensive information regarding the research design, potential risks, and benefits is paramount. This ensures that participants are well-informed about the nature of their involvement. Additionally, transparent communication allows researchers to engage positively with the communities they aim to serve, thereby promoting a culture of participation and understanding that is essential for the advancement of medical science.
Research participants deserve to have their concerns addressed and their questions answered thoroughly before, during, and after their participation. This commitment to clear communication not only enhances participant safety but also contributes to the ethical conduct of research as a whole. Regular updates regarding trial progress, any emerging risks, and results findings should be shared openly to maintain ongoing trust and assurance in the study. Thus, transparency isn’t merely a regulatory requirement; it is a binding duty that researchers owe to their participants, reflecting a mutual respect critical to the success of medical research.
Navigating Challenges in Multisite Research
Multisite research, while providing rich data for medical studies, presents unique challenges that require careful navigation through collaborative governance and logistical coordination. The SMART IRB system was developed exactly to help streamline processes that can often become cumbersome when multiple sites are involved. However, with funding cuts, the ability of these multisite collaborations to operate effectively is hampered, leading to potential delays that can stall critical advancements in treatments and therapies. Each delay could mean that breakthrough drugs or medical devices take longer to reach the patients who need them.
Furthermore, managing the complexities of multisite trials also necessitates robust IRB oversight to ensure compliance with varying institutional policies. Each site’s individual requirements must be harmonized to create a streamlined approach, ensuring that participant rights and safety are consistently safeguarded across all locations. A lack of funding impacts the resources available for joint ethical reviews, monitoring risks, and understanding diverse participant demographics. Therefore, sustaining adequate funding is essential not only for operational purposes but for maintaining the integrity of research in a shared landscape.
Research Funding and Its Crucial Role in Ethical Compliance
Research funding is intimately connected to the ethical compliance of medical studies, particularly those involving human subjects. The National Institutes of Health (NIH) and similar funding organizations provide resources that enable institutions to uphold the standards set forth by IRBs and federal regulations. When funding freezes occur, as evidenced recently, institutions may struggle to fulfill these ethical obligations adequately. This can lead to an increase in ethical conflicts, inadequate participant protections, and delays in critical oversight processes.
Moreover, financially strained institutions may be compelled to prioritize securing additional funds over stringent ethical compliance. This shift in focus can inadvertently compromise the safety of research participants. For example, insufficient funds may lead to cutbacks in training for investigators on ethical research conduct, diminishing the overall quality of IRB oversight and participant care. Hence, sustained funding is not merely an administrative concern; it is a necessary condition for upholding the ethical framework that supports patient safety in research.
The Long-term Consequences of Cuts to Research Grants
The long-term ramifications of slashing research grants extend beyond immediate funding shortages; they disrupt the entire ecosystem of medical research. A halt in funding can create a domino effect, affecting ongoing projects and leading to a significant reduction in innovation within the industry. This stagnation ultimately impacts patient safety, as potential breakthroughs that could enhance care or treatment modalities remain unexplored or undeveloped. As studies are delayed, researchers may face challenges in maintaining the momentum necessary to engage participants, potentially increasing distrust among stakeholders.
Additionally, the psychological effect on research professionals and organizations should not be underestimated. Continuous threats to funding can lead to burnout, decreased morale, and reluctance to pursue new inquiries due to uncertainty surrounding financial backing. Ultimately, as competent researchers reassess their commitments to medical studies under such pressures, the talent pool may diminish, hindering the flow of innovative approaches aimed at identifying safer and more effective healthcare solutions. Protecting research funding thus stands as a fundamental aspect of ensuring long-term patient safety and advancing medical knowledge.
Rebuilding Trust in Research Following Funding Cuts
Restoring trust in medical research after funding cuts necessitates transparent communication between institutions, researchers, and the public. The disconnection that arises when funding is frozen or cut can lead to skepticism regarding the motives of research entities and the ethics surrounding clinical trials. To rebuild this trust, institutions need to re-engage with communities, showing steadfast commitment to participant safety and ethical standards. This involves not only transparent dialogue but also actively involving community members in discussions about their concerns and expectations regarding clinical trials.
Furthermore, it is crucial to highlight the role that IRBs play in maintaining ethical oversight. By publicly sharing their measures of accountability, research institutions can reassure potential participants and the larger public that safety remains a paramount concern. Efforts to establish collaborative relationships and foster ongoing communication will be vital in dispelling lingering doubts and encouraging participation in future studies. Rebuilding trust in research is not a quick process; it requires sustained effort across all levels of the research community to demonstrate a commitment to ethical practices and participant protection.
The Role of NIH Funding in Steering Ethical Medical Research
NIH funding has historically played a critical role in steering ethical medical research practices, particularly through its support of IRB functions and participant safety initiatives. Funding from the NIH provides essential resources for ensuring that research involving human subjects adheres to ethical guidelines and legal standards. This funding not only facilitates comprehensive reviews and oversight processes but also enhances the training of research staff on ethical compliance, protecting the rights and welfare of research participants. Without NIH funding, many institutions might struggle to maintain such high standards, jeopardizing patient safety.
In addition, NIH funding allocations are instrumental in fostering collaboration among various research sites, allowing for a more integrated approach to clinical trials. When hospitals and universities have aligned resources, they can examine larger and more diverse populations, which leads to more generalizable findings and better-informed public health decisions. By maintaining steady NIH funding, the medical research community ensures the continued prioritization of ethical oversight and participant welfare, providing reassurance to research participants regarding the integrity of the trials they are involved in.
Ensuring Participant Rights in Clinical Trials
Upholding the rights of research participants is essential to fostering an ethical medical research environment. Participants must be actively informed of their rights, including the right to withdraw from a study at any time without consequence or penalty. Moreover, they should receive a clear explanation regarding the aims of the research, potential risks involved, and the measures in place to protect their privacy and data. Education about these rights empowers participants to make informed decisions and contribute to ethical conduct throughout the research process.
Furthermore, ongoing engagement with participants throughout the study reinforces their rights as stakeholders in the research process. Providing channels for reporting concerns and inquiries helps to build a sense of responsibility within research teams, as they acknowledge participants’ needs and feedback. By consistently valuing and prioritizing participant rights, researchers not only elevate the ethical standards of their work but also cultivate a renewed sense of trust within the communities they serve. Ensuring that these rights are emphasized and upheld is foundational for creating a more equitable research landscape.
Frequently Asked Questions
How does IRB oversight contribute to patient safety in medical research?
IRB oversight is crucial for patient safety in medical research as it involves reviewing and approving research proposals to ensure compliance with ethical standards and regulations. Institutional Review Boards (IRBs) assess study designs, informed consent processes, and risk mitigation strategies to protect research participants. Their role helps to maintain ethical conduct and safeguards the well-being of individuals involved in clinical trials.
What are the rights of research participants regarding patient safety in medical research?
Research participants have several rights aimed at ensuring their safety in medical research, including the right to informed consent, the right to withdraw from a study at any time, and the right to be informed about potential risks and benefits. Understanding these rights is essential for protecting patients and ensuring that their participation in clinical trials is ethical and voluntary.
How does NIH funding impact patient safety in medical research?
NIH funding plays a vital role in enhancing patient safety in medical research by supporting studies that undergo strict IRB oversight. This funding enables research institutions to develop robust safeguards for participants, bolster ethical research practices, and promote transparency, ultimately driving advancements in medical knowledge and patient care.
What happens to patient safety in medical research during funding cuts?
Funding cuts can significantly endanger patient safety in medical research by halting ongoing studies, delaying approvals for new clinical trials, and restricting the oversight capabilities of IRBs. This disruption can lead to increased risks for participants and reinforce public distrust in the research community, compromising the ethical standards essential for conducting safe and effective medical research.
How do historical events shape the current view of patient safety in medical research?
Historical events, such as unethical medical experiments and studies that disregarded participant safety, have profoundly influenced current practices in patient safety in medical research. These incidents have led to stringent regulations and the establishment of IRBs to safeguard participant rights and welfare, ensuring that the lessons from the past inform ethical oversight in clinical trials.
In what ways do clinical trials ensure the safety of research participants?
Clinical trials ensure the safety of research participants through rigorous protocols that include informed consent, regular monitoring of participant health, and the implementation of safety measures outlined by IRBs. These practices are designed to identify and mitigate risks, foster a safe research environment, and uphold the rights of those involved.
What is the SMART IRB’s role in promoting patient safety in medical research?
The SMART IRB facilitates patient safety in medical research by providing a national framework for IRB oversight across multiple sites. This system allows for streamlined review processes, enhances collaboration among institutions, and ensures consistent ethical standards are applied, ultimately safeguarding the rights and safety of research participants.
| Key Point | Details |
|---|---|
| Impact of Funding Cuts | Cuts to federal research funding have significantly disrupted patient safety efforts in medical research. |
| Role of IRBs | Institutional Review Boards (IRBs) are crucial in reviewing and overseeing research proposals to protect patients. |
| Historical Context | Past medical atrocities highlight the importance of ethical oversight in research, leading to the establishment of IRBs. |
| Effects on Research | How research studies are conducted will be impacted due to halted studies and lack of funding, risking patient trust. |
| Community and Patient Impact | The safety of patients and the integrity of clinical research require ongoing support and collaboration. |
Summary
Patient Safety in Medical Research is a critical focus area that has been jeopardized by recent funding cuts to federal research grants. The decision to halt funding not only disrupts the oversight systems necessary for protecting participants in clinical studies but also threatens the trust and safety of those involved in research endeavors. It is essential for the integrity of medical research to maintain robust funding and support for regulatory frameworks, such as IRBs, that safeguard patient rights and welfare.