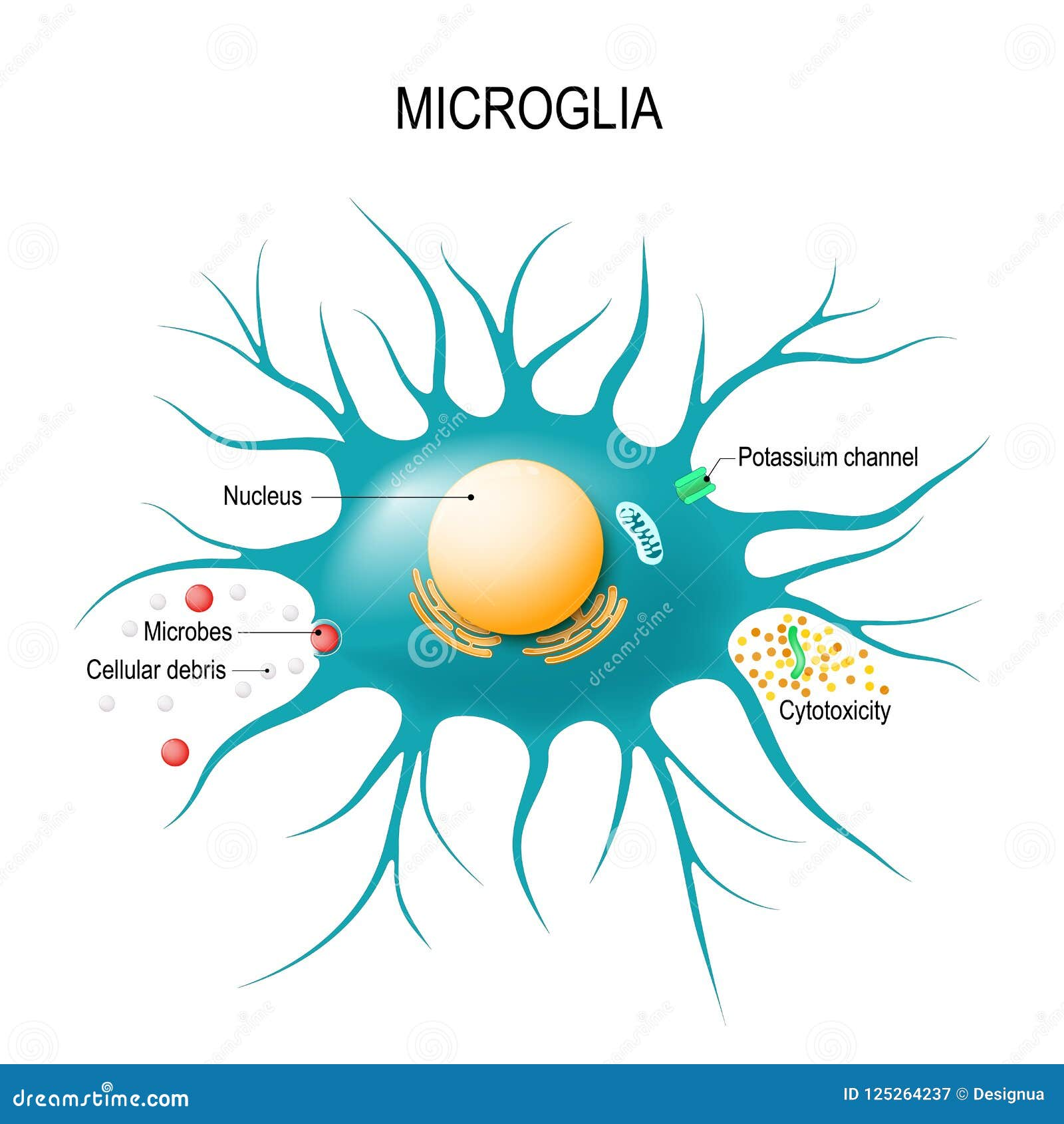
Microglial cells play a pivotal role in maintaining the health of the brain, acting as its primary immune defense. These unique cells are integral to the brain’s immune system, constantly monitoring for signs of damage or disease, particularly in the context of neurodegenerative diseases like Alzheimer’s disease. Recent research led by Beth Stevens has showcased the dual role of microglial cells in not only protecting the brain but also in their potential involvement in detrimental processes, such as synaptic pruning gone awry. This transformation in our understanding of microglial function opens new avenues for therapeutic interventions aimed at treating Alzheimer’s disease and similar conditions. With an estimated 7 million Americans affected by Alzheimer’s, innovations emerging from microglial research could profoundly impact patient care and treatment.
Known as the brain’s guardians, glial cells, and specifically microglial cells, form the core components of the central nervous system’s immune response. Their fundamental task is to surveil the brain environment for any signs of distress, making them crucial to the body’s reaction to neurodegenerative diseases, including conditions like Alzheimer’s. Exploring new dimensions of their functionality, particularly how they engage in synaptic maintenance and clearing of cellular debris, is vital as demonstrated by the groundbreaking work of researchers such as Beth Stevens. By unraveling the complexities of these immune cells, we gain deeper insights into the pathology of neurodegenerative disorders. Hence, understanding the multifaceted roles of microglial cells is becoming increasingly important in devising effective treatments for diseases characterized by cognitive decline.
Understanding Microglial Cells: The Brain’s Immune Warriors
Microglial cells play a crucial role in the brain’s immune response, acting as vigilant sentinels that monitor the health of brain tissue. These specialized cells are essential for maintaining homeostasis in the central nervous system. Their functions include phagocytosis of dead or damaged neurons and the promotion of synaptic pruning, a process critical for proper neural communication. As researchers like Beth Stevens focus on the mechanisms of microglial activity, it becomes increasingly clear how their dysfunction can contribute to neurodegenerative diseases, including Alzheimer’s disease.
Increasing evidence suggests that microglial cells may also represent a key factor in the development of various neurological disorders. When these cells become overactive or misdirected, they may erroneously prune healthy synapses, leading to cognitive decline detrimental in diseases such as Alzheimer’s. Further research into the role of microglia not only helps us understand their normal physiology but also uncovers how their pathology contributes to brain diseases, potentially paving the way for new therapeutic interventions.
The Transformative Research of Beth Stevens
Beth Stevens’ pioneering research has reshaped our understanding of microglial cells and their impact on neurodegenerative diseases. Investigating how these cells contribute to synaptic pruning, Stevens discovered that faulty microglial activity could lead to the progression of Alzheimer’s disease, significantly altering the scientific community’s approach to understanding these disorders. Her findings underscore the importance of studying the brain’s immune system to unveil new targets for treatment and to improve overall care for patients struggling with conditions like Alzheimer’s.
Stevens’ research highlights the value of a foundational understanding of brain biology in addressing complex diseases. Support from initiatives such as the National Institutes of Health has been vital in enabling her team to delve into the intricacies of microglial function. By following the thread of basic science, Stevens shows how early-stage research can eventually lead to breakthroughs in understanding disease mechanisms, which is crucial for developing effective therapies for millions of individuals impacted by neurodegenerative diseases.
Synaptic Pruning: The Double-Edged Sword in Brain Health
Synaptic pruning is an essential process during brain development where unnecessary synapses are eliminated to allow for healthier and more efficient neural circuits. This process is primarily mediated by microglial cells, which help sculpt the brain’s architecture. However, this pruning process can become detrimental when it malfunctions, leading to the loss of important synaptic connections, a mechanism observed in Alzheimer’s disease and similar neurodegenerative disorders.
The balance between adequate synaptic pruning and excessive removal is critical for optimal brain function. Stevens’ research delves into how abnormal synaptic pruning can trigger cognitive dysfunction seen in Alzheimer’s disease. Understanding the fine line that microglial cells walk in this process not only provides insight into neurodevelopmental diseases but also offers potential pathways for innovative therapies that can preserve synaptic integrity and enhance cognitive resilience in affected individuals.
Neurodegenerative Diseases and the Role of the Brain Immune System
Neurodegenerative diseases, including Alzheimer’s and Huntington’s disease, pose significant challenges to health care systems worldwide. Central to these diseases is the brain’s immune system, represented mainly by microglial cells. These cells are tasked with maintaining neural health through processes such as surveillance, cleanup, and synaptic pruning. However, in neurodegenerative conditions, dysregulation of microglial activity can lead to increased inflammation and neuronal loss, further exacerbating disease progression.
Research in neurodegenerative diseases emphasizes the importance of understanding the brain immune system’s complexities. By examining the interactions between microglial cells and neurons, scientists like Beth Stevens are uncovering how these cells contribute to both the preservation and deterioration of brain function. This knowledge harnesses the potential for developing new biomarkers and therapeutic strategies that aim to modulate microglial activity, thereby enhancing patient care and outcomes in diseases like Alzheimer’s.
Innovative Approaches to Alzheimer’s Disease Research
Innovative research approaches are vital in unraveling the complexities of Alzheimer’s disease. By shifting focus to the role of microglial cells, researchers like Beth Stevens are pioneering new methodologies that integrate basic science with translational research. This dual approach not only enhances our understanding of the disease mechanisms but also facilitates the identification of novel therapeutic avenues. For example, understanding how microglia contribute to synaptic loss could lead to targeted treatments that aim to preserve cognitive function.
Furthermore, recent advancements in genetic and molecular research techniques have allowed for a deeper investigation into the pathophysiology of Alzheimer’s disease. The ability to track changes in microglial behavior and their impact on synaptic pruning can significantly impact how researchers approach treatment options. As Stevens and her colleagues continue to explore these intricacies, their findings may lead to groundbreaking strategies to combat Alzheimer’s, providing much-needed hope for the millions affected by this debilitating disease.
The Impact of Federal Funding on Neuroscience Research
Federal funding plays a pivotal role in advancing neuroscience research, particularly in the study of diseases such as Alzheimer’s. Grants from organizations like the National Institutes of Health provide crucial resources that enable scientists to pursue investigative paths that would otherwise remain unexplored. In the case of Beth Stevens, this support has allowed her to delve into the dynamics of microglial cells and their implications for neurodegenerative diseases, laying the groundwork for innovative research that could revolutionize treatment.
The sustained investment in basic and translational science fosters a robust research environment that benefits not only individual scientists but also the broader scientific community and public health. By enabling diverse lines of inquiry into the brain’s immune system, these funds contribute to a comprehensive understanding of complex diseases and their mechanisms. In turn, this enables researchers to develop strategies that target the underlying causes of conditions like Alzheimer’s disease, ultimately improving patient outcomes and quality of life.
Future Perspectives on Alzheimer’s Disease Treatment
As the landscape of Alzheimer’s disease research evolves, future treatments are expected to integrate insights from studies of microglial cells and synaptic pruning. The identification of biomarkers that indicate dysfunctional pruning or microglial activation may lead to early diagnostic tools and intervention strategies that could modify disease progression. The research led by Beth Stevens opens up new avenues for therapeutic development that aligns with the biological underpinnings of the disease.
Incorporating advanced genomic and proteomic technologies could further enhance our understanding of Alzheimer’s pathology. Harnessing these technologies alongside behavioral studies provides a comprehensive view of how neuroinflammation and synaptic health impact cognitive function. The anticipation is that such integrative approaches will pave the way for personalized treatments that address individual variations in neurodegenerative disease progression, offering hope to patients and families affected by Alzheimer’s.
Exploring the Connection Between Microglial Cells and Cognitive Decline
The relationship between microglial cells and cognitive decline is a significant area of research in understanding Alzheimer’s disease. These immune cells are not just defenders against pathogens but also play a critical role in maintaining synaptic health, which is crucial for learning and memory. Their dysfunction can lead to neuroinflammation and increased neurodegeneration, key characteristics of cognitive decline observed in Alzheimer’s patients.
As researchers like Beth Stevens delve deeper into this connection, they seek to delineate the specific mechanisms by which microglial cells contribute to cognitive decline. Insights gathered from such studies could be instrumental in formulating interventions aimed at enhancing microglial function or mitigating their harmful effects, ultimately leading to improved cognitive health for individuals vulnerable to Alzheimer’s and other neurodegenerative diseases.
The Importance of Early Detection in Neurodegenerative Diseases
Early detection is paramount in managing neurodegenerative diseases like Alzheimer’s. Understanding the role of microglial cells and their relationship to synaptic pruning provides a framework for identifying biomarkers that may signify the onset of these diseases before significant cognitive decline occurs. Research in this area, such as that conducted by Beth Stevens, aims to develop diagnostic tools that could detect dysfunction in microglial activity, putting clinicians in a better position to initiate interventions.
Moreover, early intervention strategies that could modulate microglial activity might prevent the pathological changes associated with Alzheimer’s disease. The focus on biomarkers linked to microglial function underscores the need for continuous research that spans basic science to clinical applications. Ultimately, fostering collaboration between researchers and clinicians can improve strategies to mitigate the progression of neurodegenerative diseases, preserving cognitive function and quality of life for affected individuals.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s disease?
Microglial cells act as the brain’s immune system, monitoring for injury or disease. In the context of Alzheimer’s disease, they are responsible for removing damaged neurons and plaques, but when their function goes awry, they can contribute to neurodegeneration by excessively pruning synapses.
How do microglial cells affect synaptic pruning in neurodegenerative diseases?
Microglial cells are involved in synaptic pruning, a crucial process for normal brain development. However, in neurodegenerative diseases like Alzheimer’s, aberrant pruning by microglia can lead to the loss of critical synapses, exacerbating cognitive decline and disease progression.
What has Beth Stevens discovered about microglial cells and their relationship to Alzheimer’s?
Beth Stevens has highlighted that microglial cells are not only protective agents but also play a significant role in synaptic pruning. Her research indicates that improper functioning of these cells can contribute to the pathogenesis of Alzheimer’s disease and other neurodegenerative disorders.
Why are microglial cells considered important in the brain’s immune system?
Microglial cells are essential to the brain’s immune system because they continuously survey the environment for damage and infection. They respond to cellular distress by clearing away debris and regulating inflammation, which is crucial for maintaining brain health, particularly in conditions like Alzheimer’s.
What implications do microglial cells have for developing treatments for neurodegenerative diseases?
Understanding the functions and dysfunctions of microglial cells opens new avenues for treatments targeting neurodegenerative diseases such as Alzheimer’s. By identifying how these cells interact with neurons during synaptic pruning, researchers aim to develop strategies to restore normal microglial function and improve patient outcomes.
How do microglial cells contribute to the development of Alzheimer’s disease?
Microglial cells can contribute to Alzheimer’s disease when they mismanage synaptic pruning, leading to excessive removal of synapses. This dysregulation may stimulate neuroinflammatory processes, further damaging brain tissue and accelerating cognitive decline.
What kind of research is being conducted around microglial cells in the context of neurodegenerative diseases?
Current research focuses on elucidating the mechanisms by which microglial cells influence neurodegenerative diseases, including the identification of biomarkers, understanding their role in neuroinflammation, and developing potential therapies aimed at modulating microglial activity to halt or reverse disease progression.
Are there any known markers linked to microglial activity in Alzheimer’s disease?
Yes, researchers, including those in Beth Stevens’s lab, are working on identifying biomarkers that are associated with microglial activity. These markers could potentially indicate the state of synaptic pruning and help in diagnosing Alzheimer’s disease earlier or in monitoring the disease’s progression.
What challenges exist in studying microglial cells and their function?
One of the main challenges in studying microglial cells is their complex behavior in different conditions. Understanding the delicate balance between their protective and harmful roles in neurodegenerative diseases like Alzheimer’s requires extensive research to unravel their diverse functions over time.
How can findings about microglial cells influence future Alzheimer’s therapies?
Discoveries regarding the role of microglial cells in Alzheimer’s disease can lead to innovative therapeutic approaches, such as drugs that enhance microglial function or modulate their response. This could pave the way for targeted interventions that prevent neurodegeneration and preserve cognitive function.
| Key Points | Details |
|---|---|
| Role of Microglial Cells | Microglial cells act as the brain’s immune system, clearing out dead cells and managing synaptic pruning. |
| Research on Alzheimer’s | Beth Stevens’s research highlights how abnormal pruning by microglia can lead to conditions like Alzheimer’s and Huntington’s disease. |
| Support and Funding | The Stevens Lab receives significant support from the NIH, crucial for advancing their research. |
| Basic Science Importance | Foundational research in basic science leads to tangible advancements in understanding disease and potential treatments. |
| Impact on Care | Ongoing research has the potential to improve care for the estimated 7 million Americans with Alzheimer’s. |
Summary
Microglial cells are pivotal to understanding the immune response within the brain, particularly in the context of neurodegenerative diseases such as Alzheimer’s. These cells play a crucial role in maintaining brain health by eliminating damaged cells and regulating synaptic connections. Research led by Beth Stevens has unveiled the complexities of microglial behavior, revealing that while they are essential for brain function, their actions can inadvertently contribute to diseases when they malfunction. As scientists like Stevens continue to explore these mechanisms, the hope is that this understanding will pave the way for new therapies and better patient care for those living with Alzheimer’s.